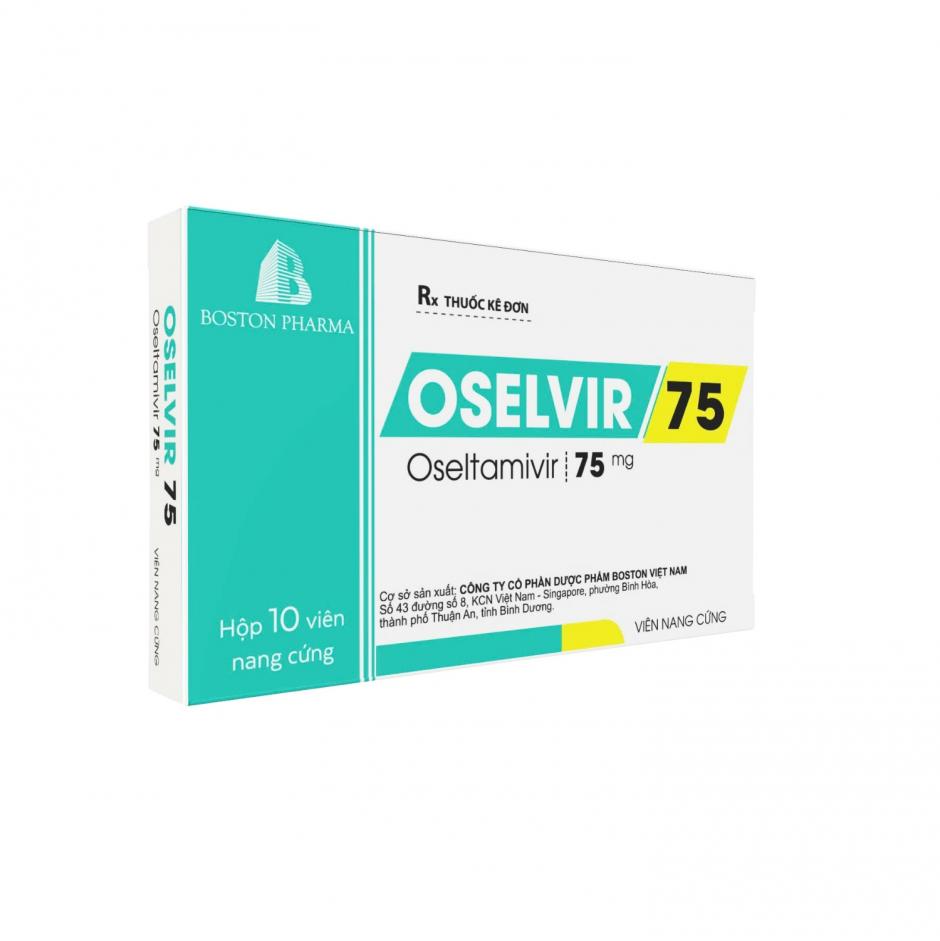
BOSFUXIM 500
202403-0310 • 6144 Views • Blister pack Al/Al. Box of 01 blister x 10 film-coated tablets with leaflet.
COMPOSITION
Each BOSFUXIM 500 film-coated tablet contains:
Active ingredient:
| Cefuroxim | 500 mg |
(*) Bioequivalence of Generic Drugs.
BOSFUXIM is indicated for the treatment of infections in adults and children from 3 months of age.
BOSFUXIM is indicated for the treatment of infections in adults and children from 3 months of age, including:
- Acute tonsillitis and pharyngitis due to Streptococcus.
- Acute bacterial sinusitis.
- Acute otitis media.
- Acute exacerbations of chronic bronchitis.
- Pyelonephritis.
- Uncomplicated skin and soft tissue infections.
- Early Lyme disease.
Consult official guidelines for appropriate use of antibiotics.
INSTRUCTIONS FOR USE:
Dosage
Adults:
|
Indication |
Dose |
|
Acute tonsillitis and pharyngitis, acute bacterial sinusitis |
250 mg twice daily |
|
Acute otitis media |
500 mg twice daily |
|
Acute exacerbations of chronic bronchitis |
500 mg twice daily |
|
Cystitis |
250 mg twice daily |
|
Pyelonephritis |
250 mg twice daily |
|
Uncomplicated skin and soft tissue infections |
250 mg twice daily |
|
Lyme disease |
500 mg twice daily for 14 days (from 10 to 21 days) |
Children (< 40 kg):
|
Indication |
Dose |
|
Acute tonsillitis and pharyngitis, acute bacterial sinusitis |
10 mg/kg twice daily up to a maximum of 125 mg twice daily |
|
Children from 2 years old with acute otitis media or more severe infections |
15 mg/kg twice daily up to a maximum of 250 mg twice daily |
|
Cystitis |
15 mg/kg twice daily up to a maximum of 250 mg twice daily |
|
Pyelonephritis |
15 mg/kg twice daily up to a maximum of 250 mg twice daily for 10–14 days |
|
Uncomplicated skin and soft tissue infections |
15 mg/kg twice daily up to a maximum of 250 mg twice daily |
|
Lyme disease |
15 mg/kg twice daily up to a maximum of 250 mg twice daily for 14 days (from 10 to 21 days) |
There is no experience with cefuroxime in children under 3 months of age.
A suspension formulation may be used for accurate dosing in children if needed.
Cefuroxime axetil tablets and cefuroxime axetil suspension are not bioequivalent and cannot be substituted on a mg basis.
Renal Impairment
The safety and efficacy of cefuroxime axetil in patients with renal impairment have not been established. Cefuroxime is primarily excreted via the kidneys. Dose adjustment of cefuroxime is recommended to account for impaired renal function. Cefuroxime is effectively removed by dialysis.
|
Creatinine Clearance (mL/min/1.73 m²) |
Half-Life (hours) |
Recommended Dose |
|
≥ 30 mL/min/1.73 m² |
1.4 – 2.4 |
No adjustment needed (standard dose 125 mg to 500 mg twice daily) |
|
10 – 29 mL/min/1.73 m² |
4.6 |
Standard dose every 24 hours |
|
< 10 mL/min/1.73 m² |
16.8 |
Standard dose every 48 hours |
|
During Hemodialysis |
2 – 4 |
Additional standard dose after each dialysis session |
Hepatic Impairment
There is no available data in patients with hepatic impairment. Since cefuroxime is predominantly excreted by the kidneys, liver impairment is considered unlikely to affect the pharmacokinetics of cefuroxime.
Administration
Administer orally. BOSFUXIM should be taken after meals for optimal absorption.
The tablets should not be crushed, and therefore BOSFUXIM tablets are not suitable for patients who cannot swallow tablets.
CONTRAINDICATIONS
Hypersensitivity to cefuroxime or any component of the tablet.
Hypersensitivity to cephalosporin antibiotics.
History of severe hypersensitivity reactions (such as anaphylaxis) to other beta-lactam antibiotics (penicillins, monobactams, or carbapenems).
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Special caution is advised in patients with a history of allergic reactions to penicillins or other beta-lactam antibiotics due to the risk of cross-sensitivity.
Severe and sometimes fatal hypersensitivity reactions have been reported with beta-lactam antibiotics. In the event of a severe hypersensitivity reaction, discontinue cefuroxime immediately and initiate appropriate emergency measures.
Assess patient history for severe hypersensitivity to cefuroxime, other cephalosporins, or any other beta-lactam antibiotics before starting treatment. Caution is warranted in patients with a history of non-severe hypersensitivity to any beta-lactam antibiotics.
Jarisch-Herxheimer Reaction
A Jarisch-Herxheimer reaction has been observed following cefuroxime axetil treatment for Lyme disease. This reaction is a direct consequence of cefuroxime axetil's antibacterial activity against Borrelia burgdorferi, the causative agent of Lyme disease. Reassure patients that this reaction is a common occurrence with antibiotic treatment for Lyme disease and usually resolves spontaneously.
Overgrowth of Non-Susceptible Microorganisms
As with other antibiotics, cefuroxime axetil may cause overgrowth of Candida fungi. Prolonged use may lead to the overgrowth of other non-susceptible bacteria (e.g., Enterococci and Clostridium difficile) and may require discontinuation of treatment.
Antibiotic-associated pseudomembranous colitis has been reported with most antibiotics, including cefuroxime, ranging from mild to life-threatening. Consider this diagnosis in patients with diarrhea during or after cefuroxime treatment. In such cases, discontinue cefuroxime and initiate specific treatment for Clostridium difficile. Avoid using anti-motility agents.
Effects on Diagnostic Tests
Positive Coombs test results related to cefuroxime use may affect cross-matching. False-negative results may occur with ferricyanide tests; therefore, glucose oxidase and hexokinase methods are recommended for blood glucose determination in patients on cefuroxime axetil.
USE IN PREGNANCY AND LACTATION
Pregnancy
Data on the use of cefuroxime in pregnant women is limited. Animal studies have not shown harm to the fetus or adverse effects on pregnancy, embryo, or fetal development, birth, or postnatal development. BOSFUXIM should only be used during pregnancy if the benefit outweighs the risk.
Lactation
Cefuroxime is excreted in breast milk in small amounts. Although it is not believed to pose a significant risk at therapeutic doses, the potential for diarrhea and mucosal fungal infections in the infant cannot be excluded. Consider discontinuing breastfeeding if necessary due to potential adverse effects. The benefits and risks should be evaluated by the responsible physician before use during lactation.
Fertility
There is no available data on the effect of cefuroxime axetil on human fertility. Animal studies do not show any effect on fertility.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINERY
No studies have been conducted on the effect of the drug on driving or operating machinery. Since the drug may cause dizziness, patients should be cautioned to avoid driving or operating hazardous machinery if they experience dizziness.
SHELF LIFE
36 months (from the date of manufacture). Do not use after the expiry date.
STORAGE CONDITIONS
Store in a dry place below 30°C, protect from light.
Blister pack Al/Al. Box of 01 blister x 10 film-coated tablets with leaflet.
Your comment



_Bosfuxim.png)